Electron Domain Formula

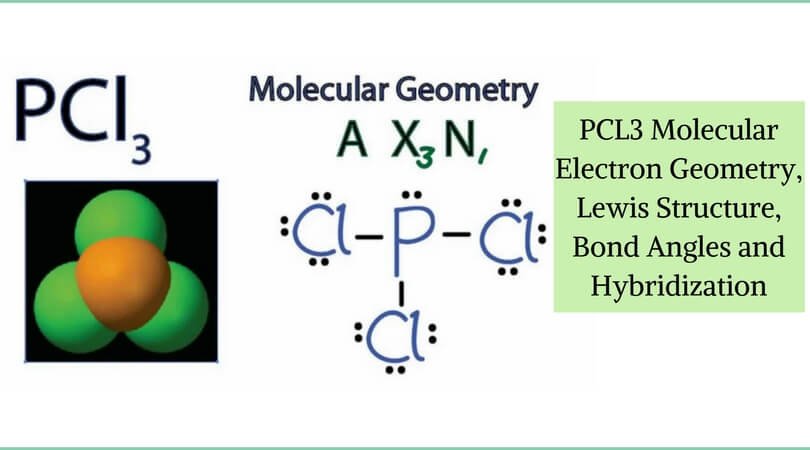
We can draw the lewis structure on a sheet of paper.
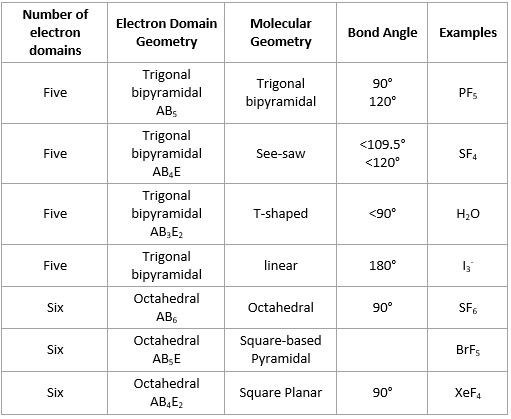
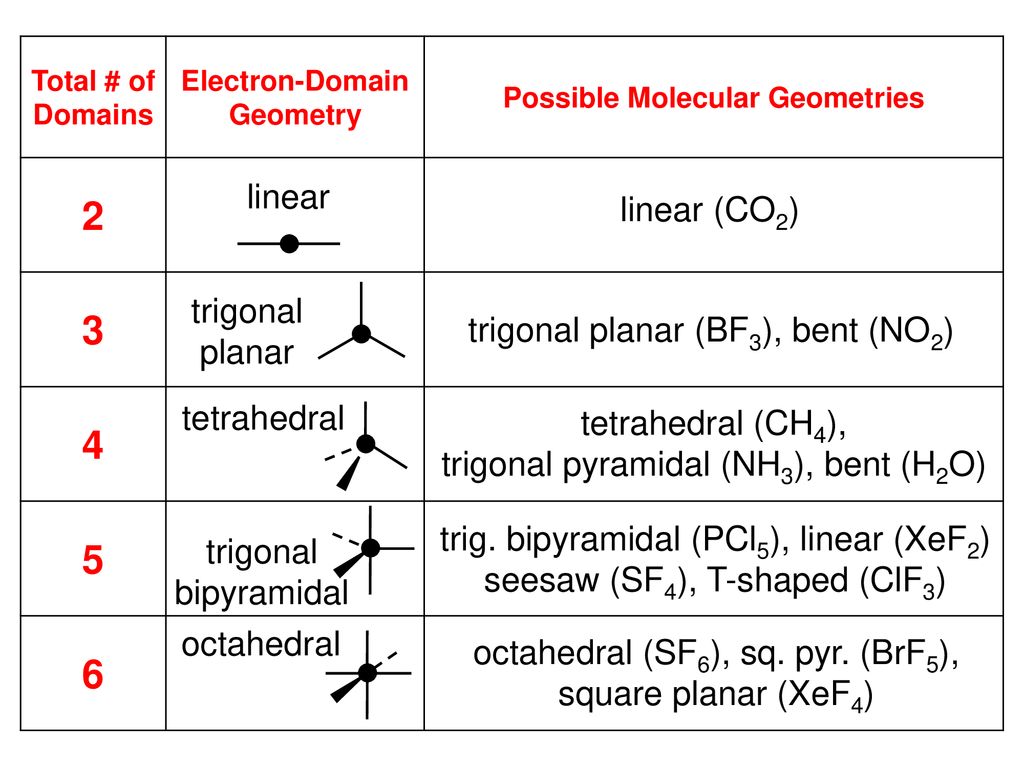
Electron domain formula. Also called charge centers also called charge centres spoiler. Trigonal bipyramidal 5 electron domains ax 5. The convention is to indicate the number of bonding electron pairs by the capital letter x the number of lone electron pairs by the capital letter e and the capital letter a for the central atom of the molecule ax n e m.
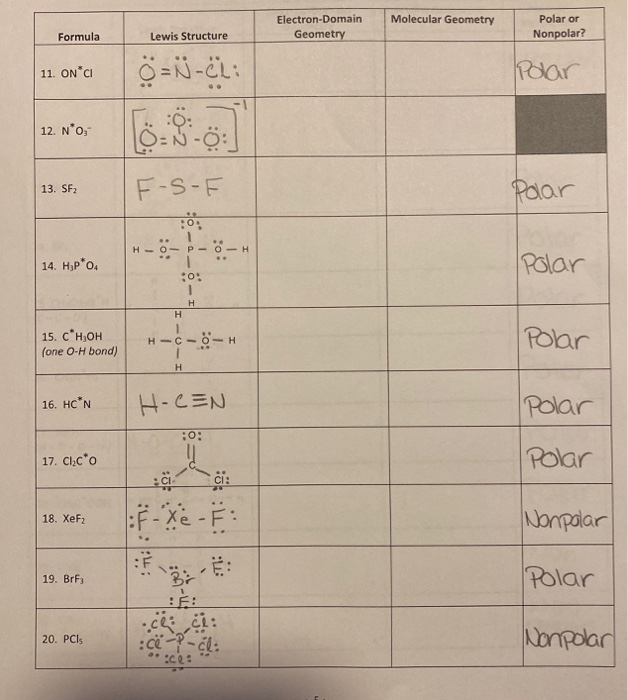
Solution for determine the structure of the following compounds include the axe vsepr notation name the molecule both with the electron domain and molecular. Tetrahedral 4 electron domains ax 4. Bf 3 alcl 3.
Electron domains may also be called electron groups. Bond location is independent of whether the bond is a single double or triple bond. In applying electron domain theory to understand this geometry we must place three points on the surface of a sphere with maximum distance between the points.
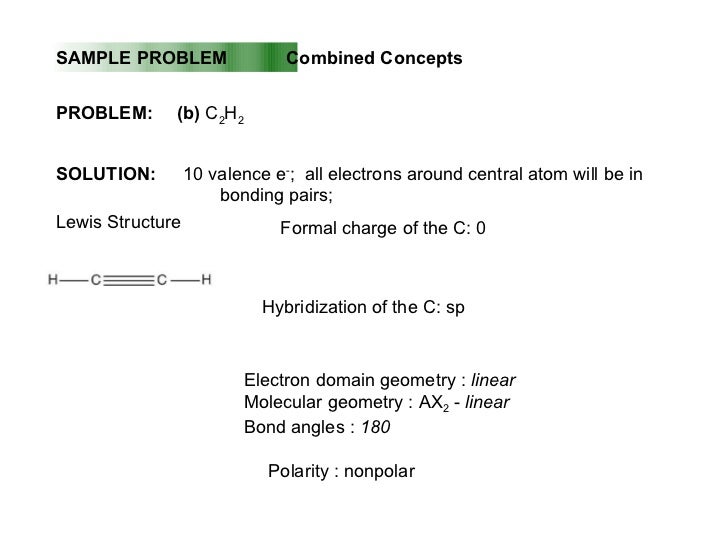
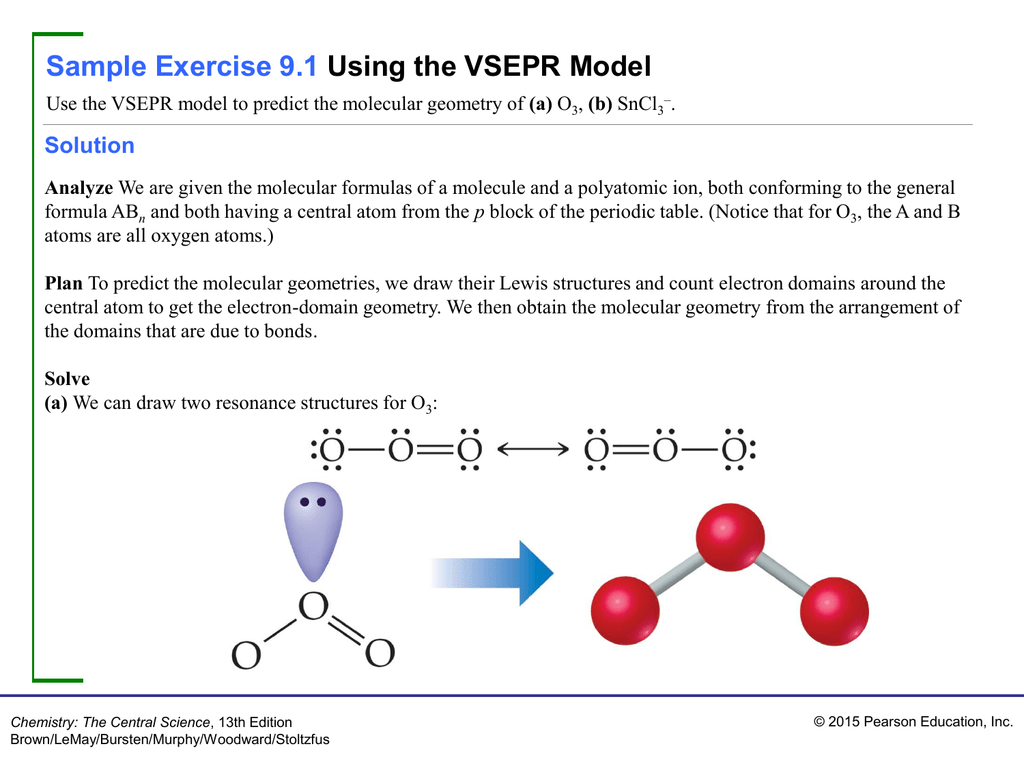
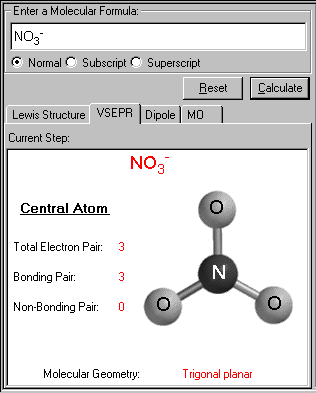
Arrangement of electron domains general molecular formula 1 molecular shape 2 examples hybrid orbitals 3 polar. For bent molecular geometry when the electron pair geometry is tetrahedral the bond angle is around 105 degrees. Valence shell electron pair repulsion theory vsepr theory enables us to predict the molecular structure including approximate bond angles around a central atom of a molecule from an examination of the number of bonds and lone electron pairs in its lewis structure.
The vsepr model assumes that electron pairs in the valence shell of a central. Trigonal planar 3 electron domains ax 3. In chemistry the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule.
Angular bent h 2 o scl 2. The most convenient way is shown here. Ch 4 sicl 4.